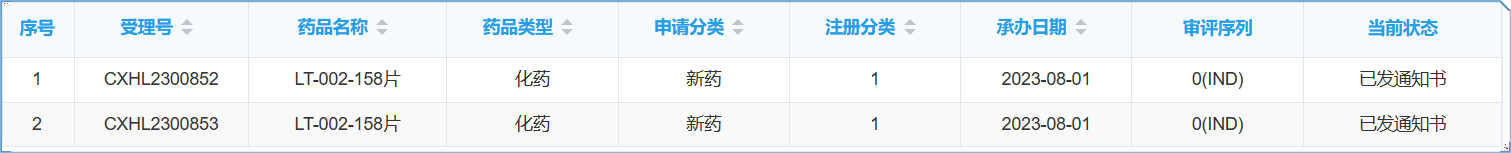
Following the U.S. FDA approval of LT-002 for the treatment of Hidradenitis Suppurativa (HS) and Atopic Dermatitis (AD) on May 24, 2023 (NCT06082323), Leadingtac received the clearance from the CDE of the Drug Review Centre of the National Drug Administration (NMPA) of China to proceed with an investigational new drug (IND) application for Phase I clinical trial of LT-002, a promising treatment for Hidradenitis Suppurativa on 18 Oct 2023. Another indication for Atopic Dermatitis is under review.
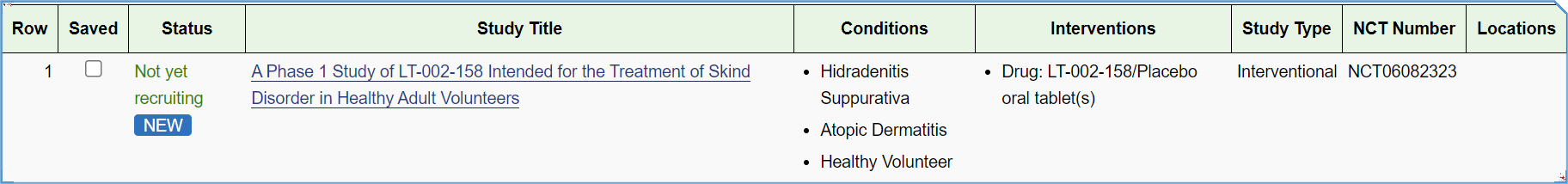
NCT Nunber:NCT06082323
“LT-002 marks a significant milestone as the first IRAK4 targeted protein degrader in China to advance into clinical trials.” said Yan Feng, Ph.D., Founder and Chief Executive Officer of Leadingtac. “In addition to LT-002, the developments of other protein degraders are in good progress, among which the second pipeline's preclinical candidate compound PCC has been selected and is planned to advance to submit IND application by the end of 2024. The lead compound for the third pipeline is being optimized and is anticipated to be submitted for IND application in Q1 2025. We would like to sincerely thank all of our employees for their tireless efforts. Looking into the future, Leadingtac will continue the commitment to helping patients suffering autoimmune disease and look forward to confer more benefits of degraders to patients.”
About IRAK4 degraders:
IRAK4 (interleukin-1 receptor-associated kinase 4) is one of the human IRAK kinase family isozymes that plays a pivotal role in protein phosphorylation as well as in cellular signaling. It receives signals from the upstream toll-like receptors (TLRs) and the interleukin-1 receptor family (IL-1R) and activates the downstream NF-κB and JNK signaling pathways, which are important for human inflammatory responses and tumors.
Kymera Therapeutics delivered an oral presentation at the European Academy of Dermatology and Venereology (EADV) symposium in Seville, Spain, May 18-20, 2023. The data showed that KT-474 administered to HS and AD patients had safety, PK and PD similar to healthy volunteers, achieved robust IRAK4 degradation in blood and skin associated with a systemic anti-inflammatory effect, and showed promising clinical activity in HS and AD.
Leadingtac's LT-002 is the first IRAK4 protein degrader in China and the second in the world to submit an IND application to the FDA following Kymera's KT-474. The molecule is developed on Nano-SPUD®, a target protein degradation drug development platform of Leadingtac. In preclinical animal models, the compound showed superior efficacy in improving skin inflammation symptoms and a favorable safety profile.
About Leadingtac:
Founded in 2019, Leadingtac is a clinical-stage biopharmaceutical company dediated to the discovery and development of First-in-class/Best-in-class small molecule innovative therapies using Protein Degradation (TPD) drug development platform with a focus on autoimmune and oncology, two therapeutic areas with great clinical unmet needs: autoimmune disease and oncology, providing breakthrough therapeutic solutions for patients. Located in Zhangjiang Science City, Shanghai, China, Leadingtac has nearly 1000 square meters of laboratory and office space, 20 employees, and has raised nearly CNY¥100 million in funding since its inception 4 years ago.