Recently, Kymera and its partner Sanofi announced plans to initiate a Phase 2 clinical trial of the IRAK4 degrader KT474/SAR444656 in HS in the fourth quarter of 2023. This news further confirms the superior safety and efficacy of the IRAK4 degrader KT-474/SAR444656 in Phase I clinical trials in healthy subjects, as well as its satisfactory clinical performance in Ic trials for the treatment of patients with hidradenitis suppurativa (HS) and atopic dermatitis (AD), and demonstrates the confidence of Kymera and its Sanofi scientific team in the promise of the IRAK4 target for the treatment of autoimmune-related diseases. Kymera and its Sanofi scientific team are confident in the future of IRAK4 targets in the treatment of autoimmune-related diseases.
According to its public information, Kymera has submitted an application for a Phase 2 clinical trial of KT-474/SAR444656 in HS on September 8, and announced the design of the Phase 2 clinical trial of KT-474/SAR444656 in HS on September 18 on its official website.
Screenshot from:U.S. National Library of Medicine
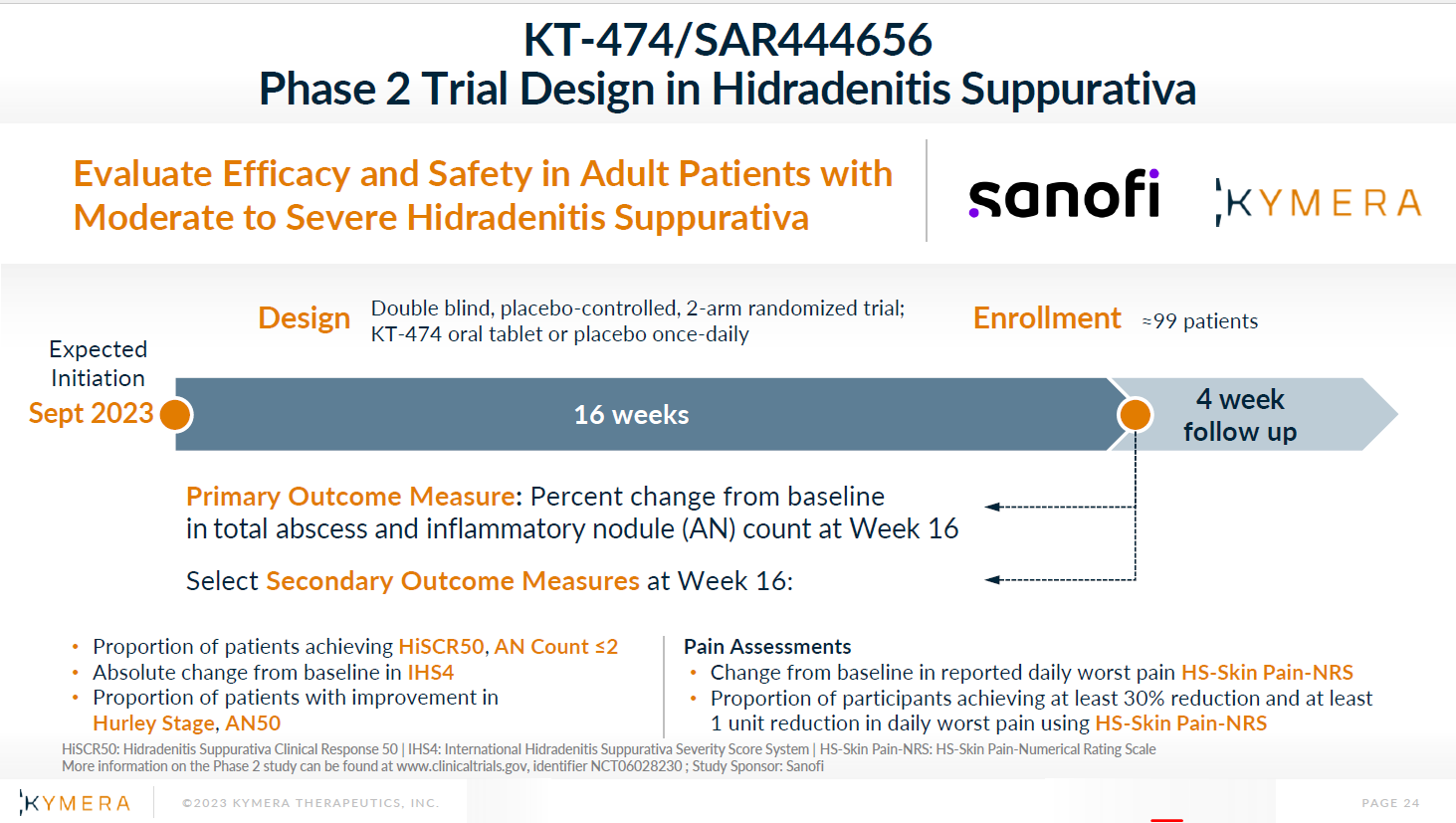
Screenshot from:Kymera fficial website
Leadingtac IRAK4 degrader LT-002 is the first IRAK4 degrader in China and the second in the world to receive FDA IND application approval after Kymera's KT-474/SAR444656. The molecule was screened based on Nano-SPUD®, Leadingtac existing protein-degrading new drug discovery platform. In preclinical animal models, the molecule demonstrated strong activity in improving skin inflammation symptoms and a favorable safety profile, and LT-002 is expected to achieve positive results in Phase I clinical trials.
About Kymera Therapeutics:
Kymera is a biopharmaceutical company pioneering the field of targeted protein degradation, a transformative approach to address disease targets and pathways inaccessible with conventional therapeutics. Kymera’s Pegasus platform is a powerful drug discovery engine, advancing novel small molecule programs designed to harness the body’s innate protein recycling machinery to degrade dysregulated, disease-causing proteins. With a focus on undrugged nodes in validated pathways, Kymera is advancing a pipeline of novel therapeutic candidates designed to address the most promising targets and provide patients with more effective treatments. Kymera’s initial programs target IRAK4, IRAKIMiD, and STAT3 within the IL-1R/TLR or JAK/STAT pathways, and the MDM2 oncoprotein, providing the opportunity to treat patients with a broad range of immune-inflammatory diseases, hematologic malignancies, and solid tumors.
Founded in 2016, Kymera is headquartered in Watertown, Mass. Kymera has been named a “Fierce 15” company by Fierce Biotech and has been recognized by both the Boston Globe and the Boston Business Journal as one of Boston’s top workplaces.
About Leadingtac:
Founded in 2019, Leadingtac is a clinical-stage biopharmaceutical company dediated to the discovery and development of First-in-class/Best-in-class small molecule innovative therapies using Protein Degradation (TPD) drug development platform with a focus on autoimmune and oncology, two therapeutic areas with great clinical unmet needs: autoimmune disease and oncology, providing breakthrough therapeutic solutions for patients. Located in Zhangjiang Science City, Shanghai, China, Leadingtac has nearly 1000 square meters of laboratory and office space, nearly 20 employees, and has raised nearly CNY¥100 million in funding since its inception 4 years ago.